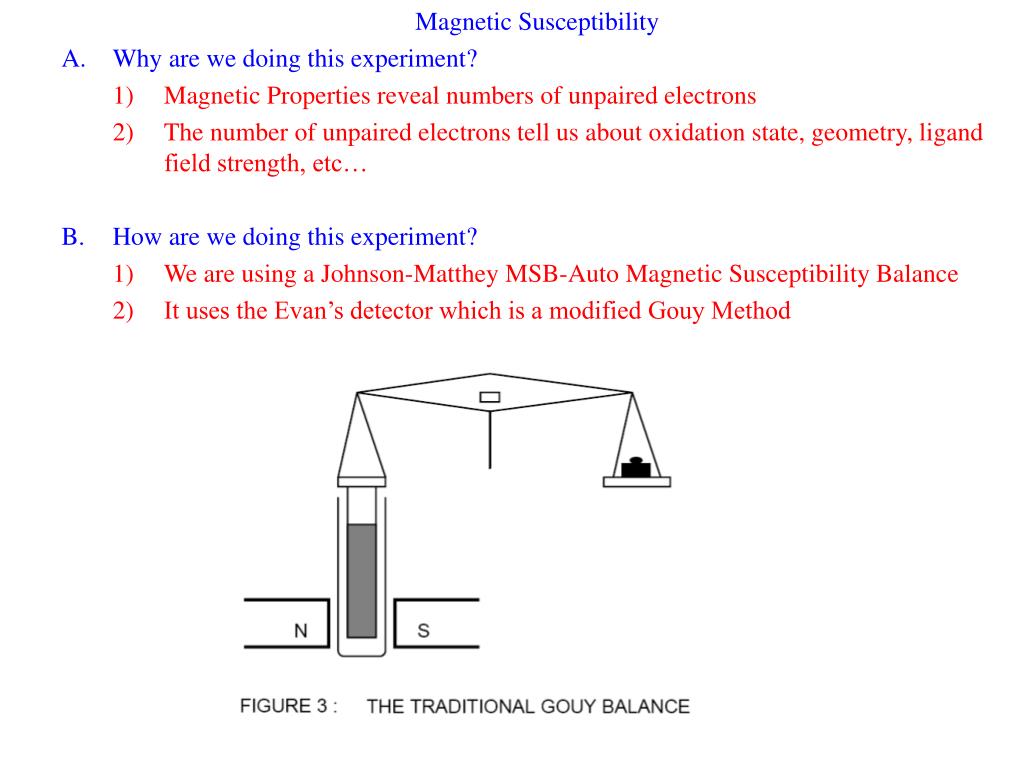
Magnetism Of Unpaired Electrons . This provides the number of. If it has unpaired electrons,. the magnetic form of a substance can be determined by examining its electron configuration: Each electron has a tiny magnetic field; Ammonia (nh 3 ) is an example of a diamagnetic compound. in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. the evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. the magnetic properties of a substance can be determined by examining its electron configuration: the magnetic properties of a substance can be determined by examining its electron configuration: paramagnetism arises due to the presence of unpaired electrons. Each electron has a tiny magnetic field; If it has unpaired electrons,. paramagnetism arises due to the presence of unpaired electrons. a compound that has unpaired electrons is paramagnetic, while one with no unpaired electrons is diamagnetic.
from www.slideserve.com
the magnetic properties of a substance can be determined by examining its electron configuration: a compound that has unpaired electrons is paramagnetic, while one with no unpaired electrons is diamagnetic. the magnetic form of a substance can be determined by examining its electron configuration: If it has unpaired electrons,. paramagnetism arises due to the presence of unpaired electrons. This provides the number of. paramagnetism arises due to the presence of unpaired electrons. Each electron has a tiny magnetic field; Each electron has a tiny magnetic field; If it has unpaired electrons,.
PPT Susceptibility Why are we doing this experiment
Magnetism Of Unpaired Electrons the magnetic properties of a substance can be determined by examining its electron configuration: If it has unpaired electrons,. This provides the number of. paramagnetism arises due to the presence of unpaired electrons. in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. the magnetic properties of a substance can be determined by examining its electron configuration: If it has unpaired electrons,. Each electron has a tiny magnetic field; paramagnetism arises due to the presence of unpaired electrons. Each electron has a tiny magnetic field; the evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. the magnetic properties of a substance can be determined by examining its electron configuration: the magnetic form of a substance can be determined by examining its electron configuration: Ammonia (nh 3 ) is an example of a diamagnetic compound. a compound that has unpaired electrons is paramagnetic, while one with no unpaired electrons is diamagnetic.
From byjus.com
what is the moment of cromium in bohr's and how many Magnetism Of Unpaired Electrons in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. the magnetic form of a substance can be determined by examining its electron configuration: If it has unpaired electrons,. Each electron has a tiny magnetic field; paramagnetism arises due to the presence of unpaired. Magnetism Of Unpaired Electrons.
From www.reddit.com
How do i learn and (mainly need help with Magnetism Of Unpaired Electrons Ammonia (nh 3 ) is an example of a diamagnetic compound. Each electron has a tiny magnetic field; If it has unpaired electrons,. the evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. the magnetic properties of a substance can be determined by examining its electron configuration: paramagnetism arises. Magnetism Of Unpaired Electrons.
From www.youtube.com
How To Determine The Number of Paired and Unpaired Electrons YouTube Magnetism Of Unpaired Electrons paramagnetism arises due to the presence of unpaired electrons. a compound that has unpaired electrons is paramagnetic, while one with no unpaired electrons is diamagnetic. Each electron has a tiny magnetic field; the magnetic properties of a substance can be determined by examining its electron configuration: This provides the number of. If it has unpaired electrons,. . Magnetism Of Unpaired Electrons.
From sciencenotes.org
vs vs Magnetism Of Unpaired Electrons paramagnetism arises due to the presence of unpaired electrons. Each electron has a tiny magnetic field; the evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron.. Magnetism Of Unpaired Electrons.
From www.slideserve.com
PPT ATOMIC STRUCTURE AND PERIODIC TRENDS PowerPoint Presentation Magnetism Of Unpaired Electrons Ammonia (nh 3 ) is an example of a diamagnetic compound. If it has unpaired electrons,. the evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. Each electron has a tiny magnetic field; paramagnetism arises due to the presence of unpaired electrons. the magnetic form of a substance can. Magnetism Of Unpaired Electrons.
From www.w3schools.blog
properties of d and f Block Elements W3schools Magnetism Of Unpaired Electrons Each electron has a tiny magnetic field; paramagnetism arises due to the presence of unpaired electrons. Each electron has a tiny magnetic field; the magnetic properties of a substance can be determined by examining its electron configuration: paramagnetism arises due to the presence of unpaired electrons. the evans method is a simple and practical method for. Magnetism Of Unpaired Electrons.
From www.researchgate.net
Schematic sketch of electronnumber parity dependent and Magnetism Of Unpaired Electrons the magnetic form of a substance can be determined by examining its electron configuration: in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. If it has unpaired electrons,. Ammonia (nh 3 ) is an example of a diamagnetic compound. paramagnetism arises due to. Magnetism Of Unpaired Electrons.
From slideplayer.com
EXPLORING ppt download Magnetism Of Unpaired Electrons paramagnetism arises due to the presence of unpaired electrons. paramagnetism arises due to the presence of unpaired electrons. the evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. This provides the number of. the magnetic form of a substance can be determined by examining its electron configuration: Each. Magnetism Of Unpaired Electrons.
From chemwiki.ucdavis.edu
7.9 Electron Spin (A Fourth Quantum Number) Chemwiki Magnetism Of Unpaired Electrons the evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. paramagnetism arises due to the presence of unpaired electrons. in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. the magnetic form of a substance. Magnetism Of Unpaired Electrons.
From byjus.com
As, is due to the presence of one or more unpaired Magnetism Of Unpaired Electrons paramagnetism arises due to the presence of unpaired electrons. the magnetic properties of a substance can be determined by examining its electron configuration: in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. If it has unpaired electrons,. If it has unpaired electrons,. . Magnetism Of Unpaired Electrons.
From www.chegg.com
Solved Part A requires unpaired Magnetism Of Unpaired Electrons paramagnetism arises due to the presence of unpaired electrons. Ammonia (nh 3 ) is an example of a diamagnetic compound. Each electron has a tiny magnetic field; This provides the number of. the magnetic properties of a substance can be determined by examining its electron configuration: Each electron has a tiny magnetic field; the magnetic properties of. Magnetism Of Unpaired Electrons.
From slideplayer.com
Chapter 8 “Covalent Bonding” ppt download Magnetism Of Unpaired Electrons the evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. the magnetic properties of a substance can be determined by examining its electron configuration: Each electron has a tiny magnetic field; Each electron has a tiny magnetic field; paramagnetism arises due to the presence of unpaired electrons. the. Magnetism Of Unpaired Electrons.
From www.numerade.com
SOLVEDOne bit of evidence that the present theory of atomic structure Magnetism Of Unpaired Electrons paramagnetism arises due to the presence of unpaired electrons. This provides the number of. paramagnetism arises due to the presence of unpaired electrons. the magnetic form of a substance can be determined by examining its electron configuration: a compound that has unpaired electrons is paramagnetic, while one with no unpaired electrons is diamagnetic. the evans. Magnetism Of Unpaired Electrons.
From www.slideserve.com
PPT Susceptibility Why are we doing this experiment Magnetism Of Unpaired Electrons Each electron has a tiny magnetic field; the magnetic form of a substance can be determined by examining its electron configuration: a compound that has unpaired electrons is paramagnetic, while one with no unpaired electrons is diamagnetic. the evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. the. Magnetism Of Unpaired Electrons.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Magnetism Of Unpaired Electrons Ammonia (nh 3 ) is an example of a diamagnetic compound. the evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. a compound that has unpaired electrons is paramagnetic, while one with no unpaired electrons is diamagnetic. Each electron has a tiny magnetic field; paramagnetism arises due to the. Magnetism Of Unpaired Electrons.
From slidetodoc.com
Chemistry Laboratory Lab 8 Experiment 12 p Magnetism Of Unpaired Electrons in chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron. Ammonia (nh 3 ) is an example of a diamagnetic compound. the magnetic properties of a substance can be determined by examining its electron configuration: If it has unpaired electrons,. paramagnetism arises due to. Magnetism Of Unpaired Electrons.
From www.researchgate.net
The spin states of the unpaired electrons separate as the Magnetism Of Unpaired Electrons Ammonia (nh 3 ) is an example of a diamagnetic compound. Each electron has a tiny magnetic field; This provides the number of. the evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. the magnetic properties of a substance can be determined by examining its electron configuration: If it has. Magnetism Of Unpaired Electrons.
From www.researchgate.net
moment of an electron. Source Magnetism Of Unpaired Electrons the evans method is a simple and practical method for obtaining the magnetic susceptibility of soluble metal complexes. the magnetic form of a substance can be determined by examining its electron configuration: paramagnetism arises due to the presence of unpaired electrons. This provides the number of. in chemistry, an unpaired electron is an electron that occupies. Magnetism Of Unpaired Electrons.